 A very cool experiment that I do is a demonstration of the properties of different gases. The air around you is actually made up of a number of gases mixed together, and we use the oxygen in it when we breathe – it is 78% nitrogen, 21% oxygen, and 1% other gases (mostly argon and water vapor). As you can see, air is actually made up of mostly nitrogen, a mostly unreactive, or inert, gas. There are lots of other gases, though, and a few of them have some really exciting properties.
A very cool experiment that I do is a demonstration of the properties of different gases. The air around you is actually made up of a number of gases mixed together, and we use the oxygen in it when we breathe – it is 78% nitrogen, 21% oxygen, and 1% other gases (mostly argon and water vapor). As you can see, air is actually made up of mostly nitrogen, a mostly unreactive, or inert, gas. There are lots of other gases, though, and a few of them have some really exciting properties.
This mixture of gas (along with everything else, really) has a property to it called density. Density is basically how much stuff is in an object. For example, if you have a brick and a block of styrofoam shaped exactly like the brick, which one is heavier? The brick, of course! That’s because it has more mass (stuff) packed into the same volume (space) as the styrofoam. That’s why styrofoam floats on water and bricks don’t – because styrofoam is less dense than water.
Now that you have an idea of what density means, we can apply that to the gases that make up air. The air around you has a density of about 1.2 g/L. That means that if you had an empty 1 liter bottle (half of a big 2-liter soda bottle), the air inside of it would weigh 1.2 grams (about the same as a paperclip).
Now, where am I going with all this? Well, we can now compare the weight of air to the weight of other gases. Helium (chemical symbol: He) is a good example – that’s what balloons at parties are filled with to make them float through the air. Helium is a very light gas and is much less dense than air – it’s density is only 0.18 g/L! Comparing that to what we found out about air earlier, we see that helium weighs almost 7 times less than air! That’s what makes helium balloons float.
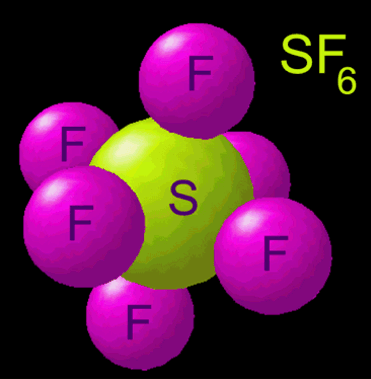 Now everyone’s probably heard of helium and how it’s lighter than air. What almost nobody has heard of is another gas with a pretty long name – sulfur hexafluoride (chemical symbol: SF6). This molecule is much more complex than the very simple helium gas. We can see from the formula that helium gas only has one atom in it – He. Sulfur hexafluoride, on the other hand, has seven atoms – one sulfur and six fluorines. This makes it much heavier, and its density is 6.16 g/L. That means it is 5 times heavier than air, and falls like a rock!
Now everyone’s probably heard of helium and how it’s lighter than air. What almost nobody has heard of is another gas with a pretty long name – sulfur hexafluoride (chemical symbol: SF6). This molecule is much more complex than the very simple helium gas. We can see from the formula that helium gas only has one atom in it – He. Sulfur hexafluoride, on the other hand, has seven atoms – one sulfur and six fluorines. This makes it much heavier, and its density is 6.16 g/L. That means it is 5 times heavier than air, and falls like a rock!
What’s really interesting is that these three gases (air, helium, and sulfur hexafluoride) are all invisible! So when you fill balloons with each of them, they all look exactly the same but weigh much different from one another. That’s one way to tell them apart, but another is to see what they do to your voice when you breathe them. Since helium is much lighter than air, it flows over your vocal cords faster and, as most people know, raises the pitch of your voice so it becomes high and squeaky! Sulfur hexafluoride, on the other hand, is much heavier than air, so what effect do you think it will have on my voice when I breathe it? Watch this clip from our first show to find out!
WARNING: This experiment can be very dangerous, and should not be tried at home. When you breathe helium (or anything else, for that matter), you aren’t breathing air and this could lead to suffocation! Bill and I are trained professionals, and we know how to do this safely. Even we only breathe tiny amounts at a time. Save yourself the risk, and watch us do it instead!
In this video clip, I have filled my green balloon with sulfur hexafluoride gas, and Gavin has filled his with regular air. Let’s see what happens when we compare the two. (This video features our new Science Siblings – Kinsey and Gavin!)


One Response to A Very Heavy Gas